Guillain barre syndrome and covid vaccine canada 133493-Can covid cause guillain barre syndrome
For those over 50, you're still at much greater risk of any adverse outcomes — GuillainBarré syndrome, blood clots or otherwise — if The CDC said in a statement on Monday that about 100 preliminary reports of GuillainBarre syndrome have been detected after 128 million doses of J&J's COVIDBackground Guillain Barre Syndrome (GBS) and Miller Fisher Syndrome (MFS) are emerging as known consequences of COVID19 infection However, there have been no reported cases with positive GM1 or

Covid 19 Tracker Amazon Adds Disclaimer To Unapproved Treatment U K Offers Booster To Immunosuppressed Ahead Of Seniors Everything Zoomer
Can covid cause guillain barre syndrome
Can covid cause guillain barre syndrome- The Medicines and Healthcare products Regulatory Agency (MHRA) is monitoring all potential side effects reported to them, including GuillainBarre Syndrome Dr June Raine, MHRA chief executive, said "Over 72 million doses of vaccines against Covid19 have now been administered in the UK, saving thousands of lives through the biggest Understanding the association between COVID19 and GuillainBarre Syndrome While large research studies are underway, overall it appears that GuillainBarre Syndrome is a rare but serious




New Covid 19 Vaccine Warnings Don T Mean It S Unsafe They Mean The System To Report Side Effects Is Working Government Executive
Reported cases of GuillainBarré Syndrome (GBS) in Canada following AstraZeneca COVID19 vaccination Data in Canada indicate a higher number of cases than would normally be expected in the general population Health Canada has updated the AstraZeneca/COVISHIELD COVID19 vaccine product monograph to include information on GBS Background There is emerging evidence that Guillain–Barré syndrome (GBS) may be associated with coronavirus disease 19 (COVID19) infection The aim of this review was to investigate the strength of the evidenceMethod The review was registered in PROSPERO (CDR) Three electronic databases, MEDLINE, PubMed, and Web of Science, and According to the CDC, there are only 39 confirmed cases of thrombocytopenia syndrome (TTS) among the 13 million people who have received the Johnson & JOhnson or Janssen COVID vaccinesThere have also been 137 reports of GuillainBarré Syndrome (GBS) among the 13 million who took the Janssen COVID vaccine As for takers of the Pfizer/BioNTech and Moderna vaccines
GuillainBarre May Be a Tragic, Though Rare, Side Effect Employers Should Promote Covid Vaccines Anyway GuillainBarre May Be a Tragic, Though Rare, Side Effect Employers Should Promote Covid Dieleman J, Romio S, Johansen K, Weibel D, Bonhoeffer J, Sturkenboom M GuillainBarre syndrome and adjuvanted pandemic influenza A (H1N1) 09 vaccine multinational casecontrol study in Europe GuillainBarre syndrome FDA flags 'small' risk with J&J jab US health officials issue new warning over rare neurological reaction for people getting the oneshot Johnson & Johnson vaccine
The nasal spray vaccine is not recommended for some groups People who SHOULD NOT get a nasal spray vaccine Children younger than 2 years of age Adults 50 years of age and older People who have had a severe or lifethreatening allergic reaction to any ingredient in the nasal spray vaccine (other than egg proteins) Physicians Offer Expanded Warning about GuillainBarré after COVID Jab TUCSON, Ariz, /PRNewswire/ The Food and Drug Administration (FDA) has issued a warning about a threetoIn July 21, the US fact sheet for the vaccine was updated to indicate that there may be an increased risk of GuillainBarré syndrome during the 42 days following vaccination 28 65 66 The European Medicines Agency (EMA) listed GuillainBarré syndrome (GBS) as a very rare side effect of COVID19 Vaccine Janssen and added a warning in



Could The Astrazeneca Vaccine Cause Guillain Barre Syndrome We Don T Know Yet But There S Minimal Cause For Concern



Http Www cdc Ca Health Info Site Documents Covid 19 Vaccine Neuromuscular Respiratory Support Clinical Guidance Pdf
Rare serious adverse events have been reported after COVID19 vaccination, including GuillainBarré syndrome (GBS) and thrombosis with thrombocytopenia syndrome (TTS) after Janssen COVID19 vaccination and myocarditis after mRNA (PfizerBioNTech and Moderna) COVID19 vaccination We are open for safe inperson care Learn more Mayo Clinic facts about coronavirus disease 19 (COVID19) Our COVID19 patient and visitor guidelines, plus trusted health information Latest on COVID19 vaccination by site Arizona patient vaccination updates Arizona, Florida patient vaccination updates Florida, Rochester patient vaccination updates Rochester and Mayo Clinic The vaccine has been approved in Canada but never used The US Centers for Disease Control and Prevention said on Monday that it has received rare reports of GuillainBarré syndrome among




10 Reasons To Put The Covid 19 Vaccine On Your Back To School List
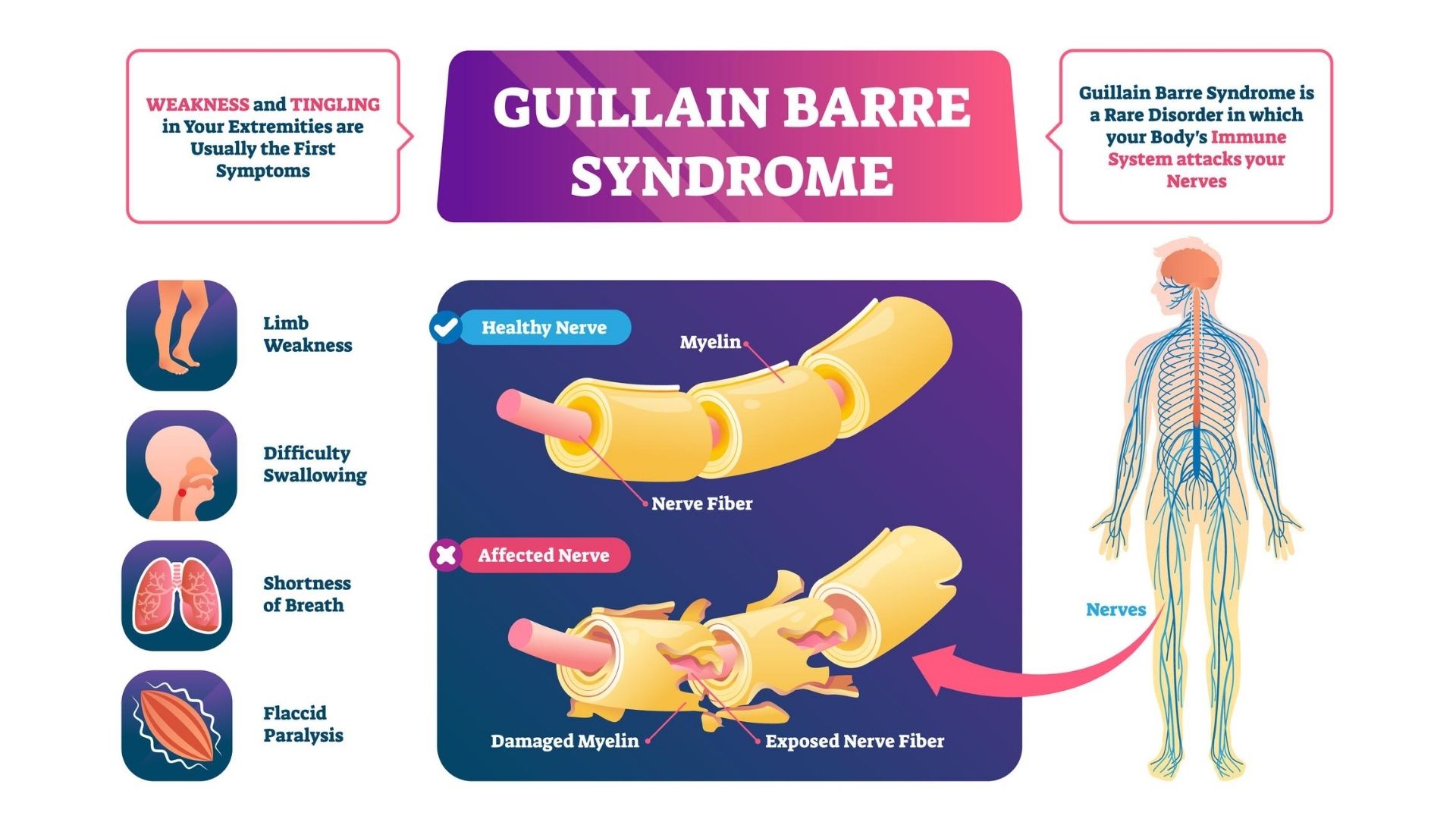



Guillain Barre Syndrome Treatment Propel Physiotherapy
He wrote a report on the issue 10 years ago and amid the COVID19 vaccine rollout in Canada, he was asked to update his work vaccine tag astrazeneca covid 19 vaccine tag GuillainBarreVery rare events of demyelinating disorders, such as GuillainBarré Syndrome, have been reported following vaccination with Janssen COVID19 Vaccine during postauthorization use Healthcare professionals should be alert to GuillainBarré Syndrome signs and symptoms to ensure correct diagnosis, in order to initiate adequate supportive care and treatment, and to rule The CDC says the agency has received about 100 reports of GuillainBarre syndrome among 128 million doses of the Johnson & JohnsonJanssen COVID19 vaccine



Canadian Pm Takes Second Dose Of Covid 19 Vaccine World News Hindustan Times



Research Rebuts Claims Linking Covid 19 Vaccines To Male Infertility Factcheck Org
GlaxoSmithKline's vaccine cash cow Shingrix has a new safety warning on its label after the FDA found an increased risk for the rare neuro autoimmune disorder GuillainBarré Syndrome in a post It's much more common to get GBS after contracting a virus or a bug than with this vaccine ― or any other vaccine, for that matter Unless AstraZeneca's Covid vaccine may trigger a nerve disorder in 'very rare' cases, according to the EU's vaccine watchdog The European Medicines Agency said today 3 cases of GuillainBarre syndrome had been reported worldwide out of 592million doses dished out



Guillain Barre Syndrome Reported Following Covid 19 Vaccination In India And England Outbreak News Today




New Covid 19 Vaccine Warnings Don T Mean It S Unsafe They Mean The System To Report Side Effects Is Working Government Executive
The role of vaccination in the development of GuillainBarré syndrome (GBS) is controversial, although cases of GBS have been reported following a wide range of vaccines A nested casecontrol study was conducted between January 11 and December 15 in three Chinese cities Four controls were matcGuillainbarreposvacinacao US Food and Drug Administration Authorizes Extending the Shelflife of Janssen's COVID19 Vaccine On 28 July 21, the US Food and Drug Administration (FDA) authorized extension of the shelflife of Janssen's COVID19 vaccine, from 45 months to 6 months, when stored between 2°C and 8°C The EMA and other regulators are already reviewing the possibility of rare blood clotting conditions with COVID19 vaccines, including AstraZeneca's GuillainBarré Syndrome is a




Janssen Covid 19 Vaccine Fact Sheet Updated With Guillain Barre Syndrome Warning
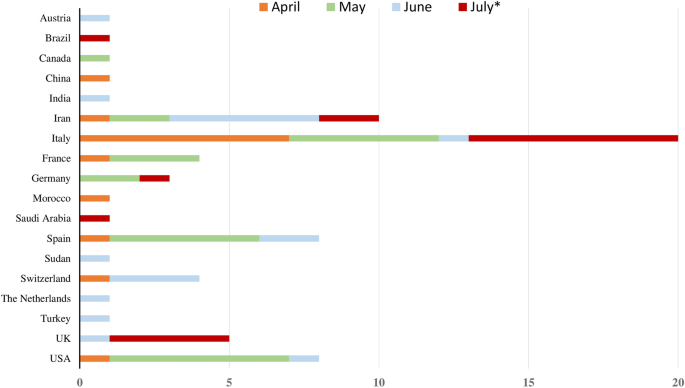



Guillain Barre Syndrome Spectrum Associated With Covid 19 An Up To Date Systematic Review Of 73 Cases Springerlink
Guillain–Barré Syndrome with Covid19 Five patients who had Guillain–Barré syndrome 5 to 10 days after the onset of Covid19 are described Three had severe weakness and an axonal pattern onIn 1976, a massive swine flu vaccination program was halted when doctors began diagnosing a similar disorder, GuillainBarré syndrome, in people who received the vaccine GuillainBarré Syndrome What NIIP did not and could not survive, however, was the second blow, finding cases of GuillainBarré syndrome (GBS) among persons receiving swine flu immunizations As of 1976, >50 "antecedent events" had been identified in temporal relationship to GBS, events that were considered as possible factors in its cause



Fda Lists Rare Neurological Condition As Potential Side Effect Of J J S Covid 19 Vaccine Pmlive



Fda Adds Guillain Barre Warning To J J Vaccine What To Know Time
Acute demyelinating inflammatory polyneuropathy (AIDP) is the most common type of GuillainBarré syndrome (GBS) in Europe, following several viral and bacterial infections Data on AIDPpatients associated with SARSCoV2 (coronavirus2) infection are scarce We describe the case of a 54yearsold C Article content Europe's medicines regulator has added an extremely rare nervedamaging disorder, GuillainBarré syndrome, as a possible sideeffect of AstraZeneca's COVID19 vaccine, regular safety updates from the watchdog showed on Wednesday A Houston woman who developed the rare neurological condition GuillainBarré syndrome (GBS) after receiving the onedose Johnson & Johnson COVID19 vaccine said she would get the shot again




Here S What We Know About Guillain Barre Syndrome And Vaccines The New York Times




Applications Open For Federal Vaccine Injury Compensation Cbc News
New Delhi Eleven people who received the AstraZenecaOxford COVID19 vaccine have developed a rare neurological disorder called GuillainBarre syndrome, clinicians in Health Care FDA adds GuillainBarre syndrome warning to J&J Covid shot About 100 suspected cases of GBS — among 128 million people who have gotten the J&J shot — have been identified in the61 COVID19 vaccines description and general safety considerations for GACVS Global Advisory Committee on Vaccine Safety GBS GuillainBarré syndrome GMP Good manufacturing practices GVAP Global vaccine action plan HCW Health care worker SARSCoV2 Severe acute respiratory syndrome coronavirus 2 SKG Significant knowledge gap




Npyt2cnqlkn1dm



Coronavirus Vaccine Side Effects Guillain Barre Syndrome How Risky Is Guillain Barre Syndrome The Nervous System Disorder Identified As A Covid 19 Vaccine Side Effect
Key Summary Points Guillain–Barré syndrome (GBS) is an autoimmune disorder of the peripheral nervous system that typically develops following infection The association of emerging infectious diseases, such as Zika virus infection and coronavirus disease 19 (COVID19), with GBS is a topic of debateCauses GuillainBarré syndrome is thought to be caused by a problem with the immune system, the body's natural defence against illness and infection Normally the immune system attacks any germs that get into the body But in people with GuillainBarré syndrome, something goes wrong and it mistakenly attacks the nerves Cases of GuillainBarre syndrome following the Johnson & Johnson vaccine are extremely rare There have been 100 reports of GBS out




We Are Not Anti Vaxxers Concerns Over Side Effects Research Among Main Reasons Some Canadians Are Not Getting Covid 19 Vaccine



It S A Razor S Edge We Re Walking Inside The Race To Develop A Coronavirus Vaccine Coronavirus The Guardian
Read Exclusive summary of Covid19 vaccine concerns The list includes GuillainBarre syndrome, an autoimmune disorder which can be deadly, causing irrecoverable paralysis The FDA recently warned that the Johnson and Johnson Covid19 vaccine may be linked to numerous cases of GuillainBarre syndrome The FDA added a warning that Johnson & Johnson's COVID19 vaccine may trigger GuillainBarré syndrome (GBS) in a small number of people in a letter sent to the manufacturer on Monday Of the 125 By December, the Swine Flu vaccination program was suspended when people started to develop GuillainBarré Syndrome, a rare neurological condition whose risk was seven times higher in people who




Covid 19 Why Some Canadians Are Choosing Not To Get Vaccinated Ctv News




Was The Covid 19 Vaccine Rushed And What Are The Side Effects Cbc Asked An Expert Cbc News
Press Release On 13 and July 21, the COVID19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS) met virtually to discuss rare reports of GuillainBarré SyndromeA 57yearold man presented with a progressive flaccid symmetrical motor and sensory neuropathy following a 1week history of cough and malaise He was diagnosed with GuillainBarré syndrome secondary to COVID19 and started on intravenous immunoglobulin He proceeded to have worsening respiratory function and needed intubation and mechanical ventilationSeek medical attention right away if you develop these symptoms in the days following vaccination GuillainBarré syndrome (GBS) is a neurological disorder where inflammation of peripheral nerves causes rapid muscle weakness and can sometimes lead to paralysis This has been reported very rarely after vaccination with Janssen COVID19 Vaccine
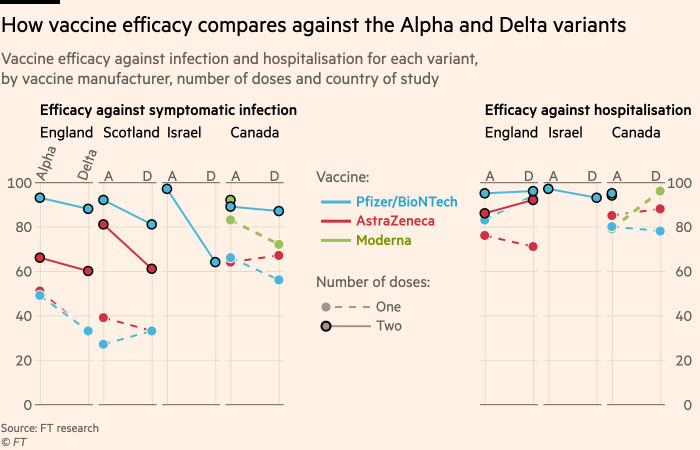



Coronavirus Cdc Says Reopening Us Schools A Priority As It Happened Financial Times




Learn More About Covid 19 Vaccines From The Fda Fda
The team, from the Mondino Foundation in Italy, have warned that some patients are developing Guillain–Barré syndrome after the onset of COVID19 GuillainBarré syndrome is aGuillainBarre syndrome (GBS) is an extremely rarely reported side effect and risk following any vaccination We describe a case of GBS following vaccination against COVID19, in the absence of any further triggers There have been reports of GBS after other Covid19 vaccines, but FDA has said it does not yet see a link and the illnesses could be just coincidental Public health officials say the benefits of Covid19 vaccines outweigh the risks among the eligible population




Federal Compensation Not Ready Yet For Vaccine Injuries Deaths Cbc News




Case Reports Describe Unusual Guillain Barre Variants Following Covid 19 Vaccination
AS the world scrambles to get vaccinated against coronavirus disease 19, or Covid19, there is also a minority, including reputable doctors and scientists, raising concern, if not alarm, over the rush to get jabbed In news and social media posts and videos, these vaccinewary voices cite reports of adverse and even fatal events following Covid




Guillain Barre Syndrome And Johnson Johnson Vaccine What You Need To Know The Independent




Underlying Medical Conditions And Covid 19 Vaccines When Getting A Coronavirus Shot May Not Be Advisable Ctv News



Latest Pfizer Will Soon Seek Emergency Approval Of Vaccine For Kids 5 And Older




Britain Germany In Role Reversal On Astrazeneca Vaccine Risks Reuters




Cureus Neurological Complications Of Covid 19 Guillain Barre Syndrome Following Pfizer Covid 19 Vaccine




Study Finds No Link Between Covid 19 Guillain Barre Syndrome Cidrap




Covid 19 Vaccine Information Hamilton Family Medicine



Rarest Of Covid Vaccine Reactions Guillain Barre Syndrome Whyy




J J Astrazeneca Explore Covid Vaccine Changes Due To Clots Wsj Coronavirus Pandemic News Al Jazeera



Covid 19 Vaccine Safety Weekly Report On Side Effects Following Immunization Canada Ca




What Side Effects Could You Get From Moderna Pfizer Coronavirus Vaccines




What Covid Vaccines Are Related To The Guillain Barre Syndrome What Are The Cdc Guidelines As Com




Canadian Immunization Guide Chapter On Influenza And Statement On Seasonal Influenza Vaccine For 21 Canada Ca




Seasonal Influenza Vaccine And Guillain Barre Syndrome Neurology




Fda Warns Johnson Johnson Covid 19 Vaccine Linked To Rare Neurological Reaction National Globalnews Ca




Janssen Covid 19 Vaccine Fact Sheet Updated With Guillain Barre Syndrome Warning
/cloudfront-us-east-1.images.arcpublishing.com/tgam/NA5AO3D6ONIL3M6ELSBSEQ62LQ.jpg)



Opinion Canada S Vaccine Legacy Influenza Polio And Covid 19 The Globe And Mail




The J J Vaccine For Covid 19 What To Know




Who Warns Against Mixing Covid Vaccines What You Should Know Wfla




What Covid Vaccines Are Related To The Guillain Barre Syndrome What Are The Cdc Guidelines As Com




Rnr5lgyyqsgz1m




What Research Says About Covid 19 Vaccine Side Effects In Canada And Number Of Lives Saved By Vaccines In U S Georgia Straight Vancouver S News Entertainment Weekly
:strip_exif(true):strip_icc(true):no_upscale(true):quality(65)/d1vhqlrjc8h82r.cloudfront.net/04-05-2021/t_689c266fe386406b9f7e275e79d31dfd_name_image.jpg)



Local Teen Diagnosed With Guillain Barre Syndrome Questions Covid 19 Vaccine After Receiving First Dose




J J Covid Vaccine May Pose Small Possible Risk Of Rare Neurological Syndrome Cdc Says Cbc News
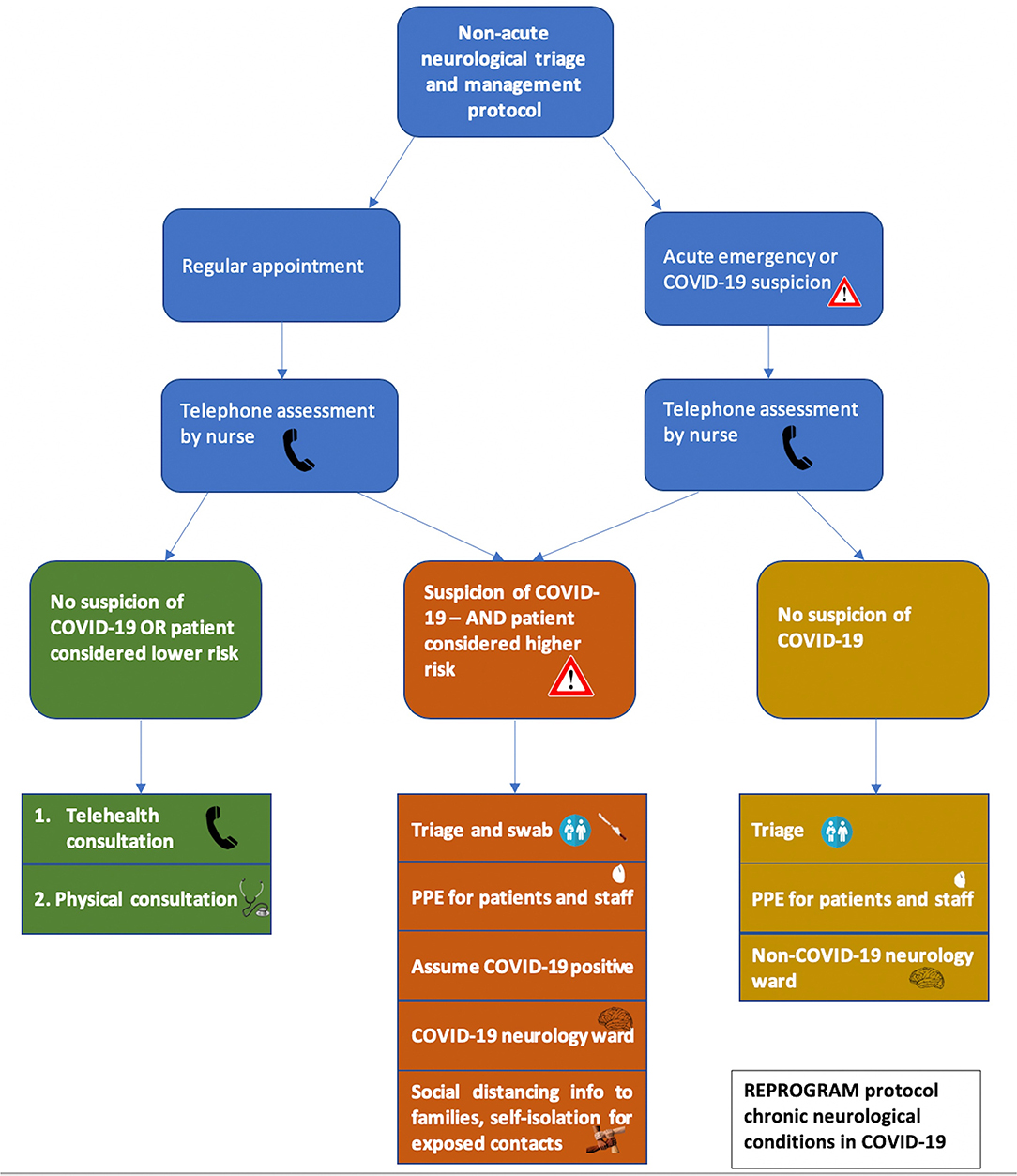



Frontiers Chronic Neurology In Covid 19 Era Clinical Considerations And Recommendations From The Reprogram Consortium Neurology




Coronavirus Vaccines What You Should Know About The Side Effects National Globalnews Ca




Covid 19 Vaccines Serious Adverse Events Explained



New Covid 19 Vaccine Warnings Don T Mean It S Unsafe They Mean The System To Report Side Effects Is Working




Guillain Barre Experts Clarify Covid 19 Vaccine Confusion With Open Letter To Dr A S Fauci




Coronavirus Vaccines What You Should Know About The Side Effects National Globalnews Ca




Ontario Leader Blames Pfizer For Covid 19 Vaccine Delays Health News Us News




Az J J To Research Modifications To Covid 19 Vaccines Over Rare Blood Clot Issues Pmlive



Fda Adds Warning To J J Vaccine For Possible Link To Rare Neurological Disorder
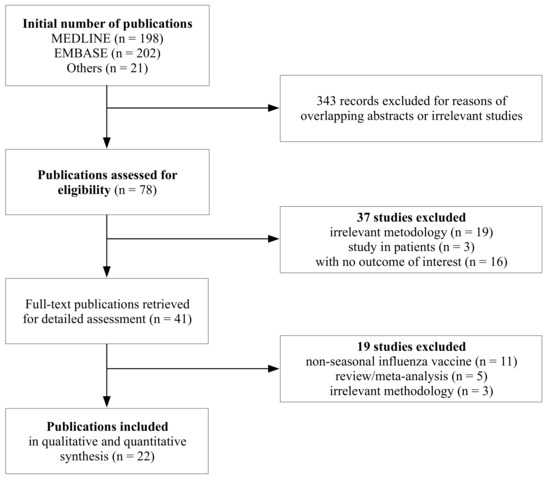



Vaccines Free Full Text Is An Increased Risk Of Developing Guillain Barre Syndrome Associated With Seasonal Influenza Vaccination A Systematic Review And Meta Analysis Html




Guillain Barre Syndrome Reported Following Covid 19 Vaccination In India And England Outbreak News Today




Covid 19 Tracker Amazon Adds Disclaimer To Unapproved Treatment U K Offers Booster To Immunosuppressed Ahead Of Seniors Everything Zoomer




Q A Guillain Barre Syndrome In Patients With Covid 19 Requires More Research



What Are The Symptoms Of Guillain Barre Syndrome Should I Still Get The J J Vaccine Marketwatch
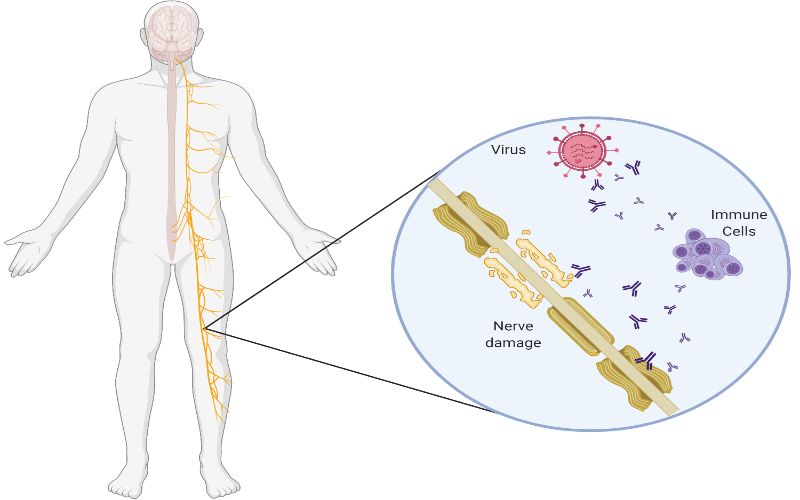



No Association Between Covid 19 And Guillain Barre Syndrome Ucl News Ucl University College London




We Need A Covid 19 Vaccine Let S Get It Right The First Time Wired




Guillain Barre Syndrome And Its Variants As A Manifestation Of Covid 19 A Systematic Review Of Case Reports And Case Series Journal Of The Neurological Sciences




Emerging Infectious Diseases Vaccines And Guillain Barre Syndrome Koike 21 Clinical And Experimental Neuroimmunology Wiley Online Library




Covid 19 Tracker Merck Eyes Year End Fda Nod For Oral Drug Cdc Biden Admin Said To Clash On Boosters Fiercepharma




What To Know About The Johnson Johnson Covid Vaccine And Guillain Barre Syndrome Huffpost Life



Vaccine Safety Gvn



Covid Vaccine What You Need To Know About Vaccine Safety c News



Www Jns Journal Com Article S0022 510x 2 Pdf



New Vaccines For A Novel Virus Insights From Coronavirus And Vaccine Experts Findings University Of Michigan School Of Public Health Epidemic Flu Infectious Disease Vaccines Pharmaceuticals
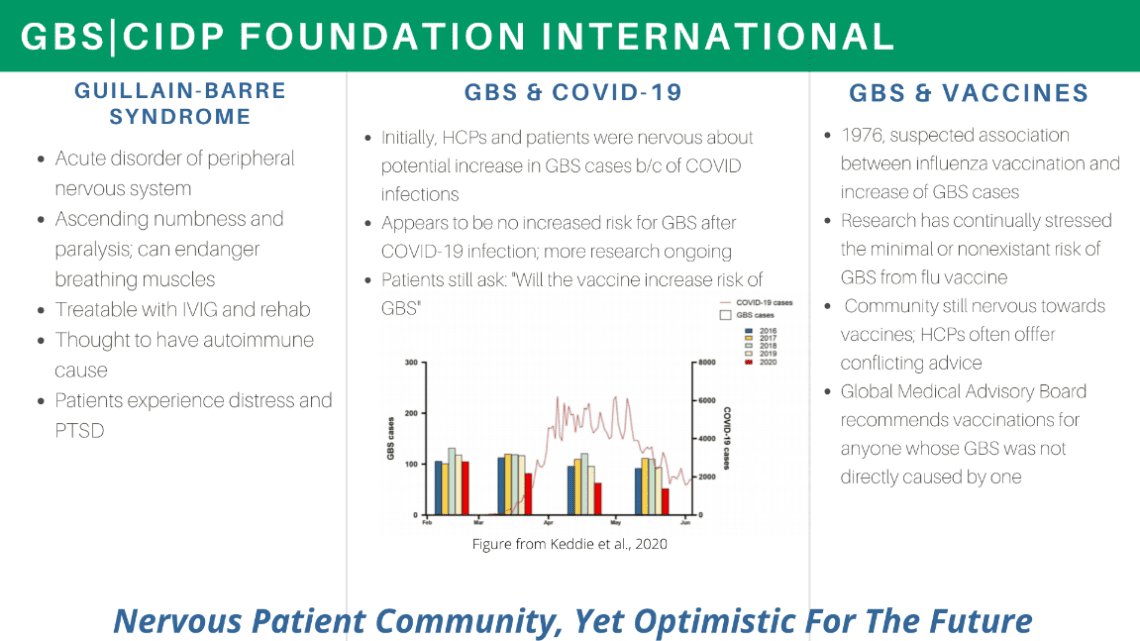



Gbs Cidp Foundation Staff Patients Collaborate With Fda Gbs Cidp Foundation International




Guillain Barre Syndrome The Lancet




Canadian Pm Opts For Moderna Vaccine For Second Dose World News Hindustan Times



Eu Reviews Reports Of Rare Nerve Disorder After Astrazeneca S Covid 19 Shot Reuters




Covid Vaccine Safety System Has Gaps That May Miss Unexpected Side Effects Experts Say




Stakeholder Call Guillain Barre Syndrome Gbs Covid 19 Vaccine Updates 7 15 21 Youtube




Guillain Barre Syndrome And The Johnson Johnson Vaccine What To Know The New York Times



Coronavirus J J Talking To Us Regulators About Rare Neurological Side Effect Of Vaccine As It Happened Financial Times




What To Know About Guillain Barre And The Covid Vaccine Cleveland Clinic



Covid Child Jabs Halted In Trial As Adult Clot Link Probed c News



Could The Astrazeneca Vaccine Cause Guillain Barre Syndrome We Don T Know Yet But There S Minimal Cause For Concern



Get The Facts About Covid 19 Vaccines Mayo Clinic



Cureus Neurological Complications Of Covid 19 Guillain Barre Syndrome Following Pfizer Covid 19 Vaccine




Fda Plans To Warn J J Covid 19 Vaccine Raises Risk Of Rare Neurological Condition




Fda Adds Guillain Barre Syndrome Warning To J J Covid Shot Politico




Cdc People With Weakened Immune Systems Autoimmune Conditions Or Previously Diagnosed With Guillain Barre Syndrome Or Bell S Palsy May Receive An Mrna Covid19 Vaccine However There Are Limited Safety Data Learn




Is It Safe To Get The Covid 19 Vaccine If You Have Guillain Barre Syndrome



Covid 19 Vaccines Centre For Effective Practice Digital Tools




No Proof Covid Vaccines Can Trigger Guillain Barre Syndrome Health News Us News




Astrazeneca Covid Vaccine Linked To Rare Neurological Disorder In India Uk The Economic Times



Covid 19 Vaccine Experts Call For Injury Compensation Program Ctv News



Flu Shots And Vaccinations Gbs Cidp Foundation International



Why The Push For A Quick Coronavirus Vaccine Could Backfire Politico




Risk Of Guillain Barre Syndrome After Seasonal Influenza Vaccination And Influenza Health Care Encounters A Self Controlled Study The Lancet Infectious Diseases



Covid Vaccine Safety System Has Gaps That May Miss Unexpected Side Effects Experts Say



Cureus Neurological Complications Of Covid 19 Guillain Barre Syndrome Following Pfizer Covid 19 Vaccine




Guillain Barre Syndrome Fda Flags Small Risk With J J Jab Coronavirus Pandemic News Al Jazeera




Acog Releases Updated Covid 19 Vaccine Recommendations For Obstetric Gynecologic Care Rheumatology Advisor
コメント
コメントを投稿